This site is intended for Healthcare Professionals only. if you are a member of the general public, click here
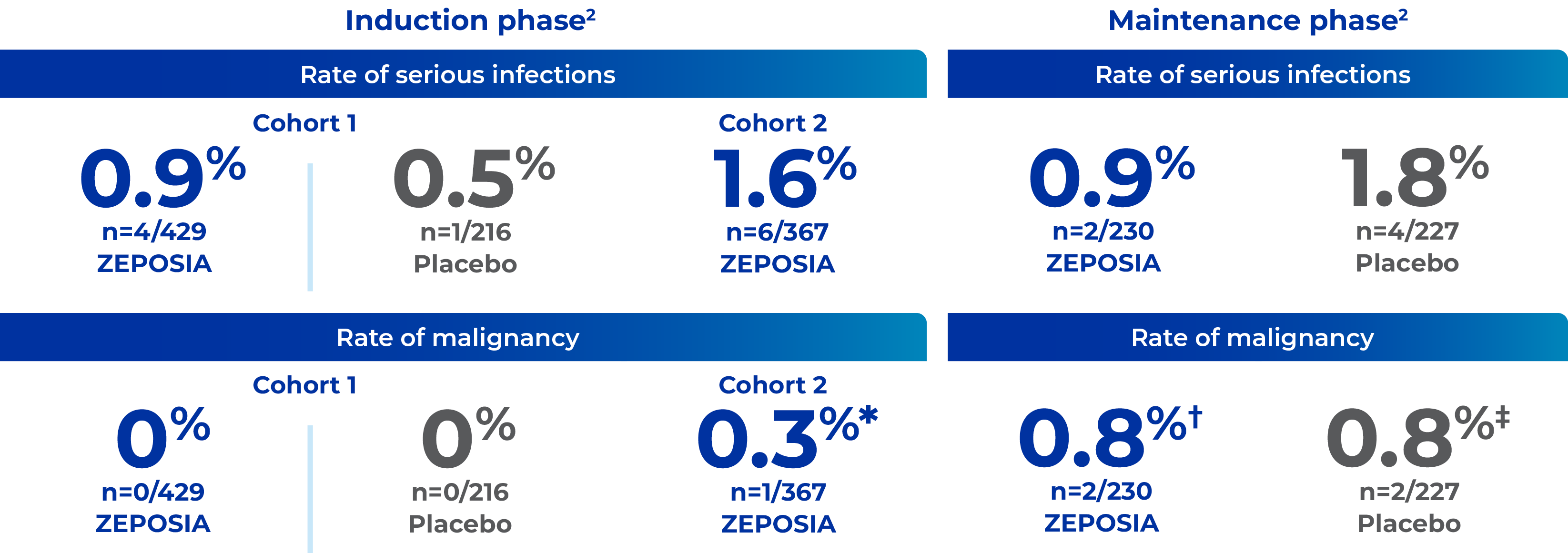
Rates of serious infection and malignancy in the TRUE NORTH study1,2
You are advised to read the study design information before these safety outcomes
 Pinch & zoom to explore
Pinch & zoom to explore
- Herpes zoster was reported in 2.2% of patients receiving ZEPOSIA vs 0.4% receiving placebo in the maintenance phase1 — No cases were serious or disseminated
- All malignancies in ZEPOSIA patients were found by investigators to be unrelated to the study drug2
Please refer to the ZEPOSIA Summary of Product Characteristics for further safety information
ZEPOSIA has an immunosuppressive effect that predisposes patients to a risk of infection, and may increase the risk of developing malignancies. Physicians should carefully monitor patients, especially those with concurrent conditions or known factors.
Direct comparison can not be made between ZEPOSIA and placebo as no statistical test of comparisons have been made.
*1 patient had basal cell carcinoma.2
†1 patient had basal cell carcinoma; 1 patient had rectal adenocarcinoma.2
‡1 patient had breast cancer; 1 patient had adenocarcinoma of the colon.2
OLE, open-label extension; PT, preferred term; SOC, standard of care; UC, ulcerative colitis.
References
- Zeposia (ozanimod) Summary of Product Characteristics, 2022.
- Sandborn WJ et al. NEJM 2021; 385(14):1280–1291.

