This site is intended for Healthcare Professionals only. if you are a member of the general public, click here
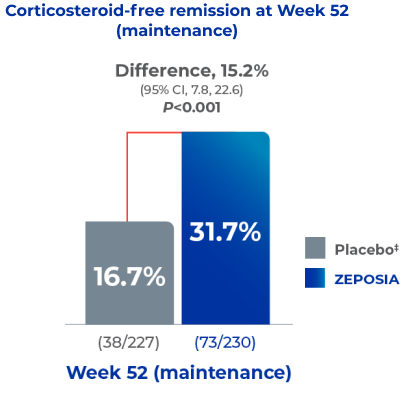
With ZEPOSIA, significantly more patients achieved corticosteroid-free remission (secondary endpoint)* at 52 weeks vs. placebo1
You are advised to read the study design information before these efficacy outcomes
Secondary endpoint: Corticosteroid-free remission (remission with no steroid use for ≥12 weeks) at week 52.1*
 Pinch & zoom to explore
Pinch & zoom to explore
In the TRUE NORTH study:
- Nearly twice as many patients
who received ZEPOSIA achieved corticosteroid-free remission at 52 weeks vs. placebo1
Adapted from Sandborn WJ et al. 2021.
Key secondary efficacy endpoints were assessed in a pre-specified hierarchical testing procedure. The ranked secondary endpoints for the maintenance period (52 weeks) were percentages of patients with a clinical response, endoscopic improvement, maintenance of clinical remission, corticosteroid free remission, mucosal healing, and durable remission.1
*Corticosteroid-free remission was defined as clinical remission at 52 weeks without receipt of glucocorticoids for at least 12 weeks.
CI, confidence interval.
References
- Sandborn WJ et al. NEJM 2021; 385(14):1280–1291.

