S1P1 receptor
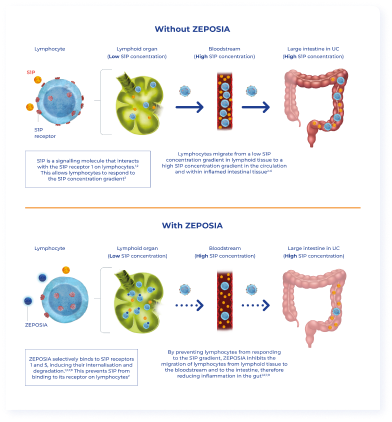
The regulation of lymphocyte migration is one of the best characterised functions of the S1P pathway, which is mainly mediated by the interaction of S1P with the S1P1 receptor. S1P1 is the most widely expressed S1P receptor, being commonly found on endothelial cells and lymphocytes.3 The interaction of S1P to the S1P1 receptor is what drives the migration of lymphocytes from the spleen and lymphoid tissues into the systemic circulation.3,4 This migration of lymphocytes across the body is achieved through a S1P gradient, with the S1P concentration being substantially higher in the circulation than in lymphoid tissues and organs.2,4
S1P2 and S1P3 receptors
S1P2 and S1P3 mediate vascular, intestinal, bronchial, and bladder smooth muscle vasoconstriction. Modulation of the S1P2 receptor by S1P is hypothesised to have opposite functions to S1P1 modulation, with a pro-inflammatory role expected. Pharmacological agents targeting these receptors have been associated with side effects such as hypertension and renal ischaemia reperfusion injury.4

S1P4 receptor
S1P4 regulates immune system function by guiding cell migration and differentiation of specific immune cells. The interaction of S1P with S1P4 mediates immunosuppressive effects by inhibiting secretion of effector cytokines while increasing the production of the anti-inflammatory molecule IL-10. In addition, S1P4 expression on dendritic cells plays a role in regulation of Th17 cells and IL-27 production, thereby allowing regulatory T cells to suppress cytotoxic T (CD8+ T) cells. S1P4 receptor expression also enhances trafficking of neutrophils from inflamed tissues to the draining lymph node.4

S1P5 receptor
The S1P5 receptor plays a role within the immune system by helping to guide natural killer cells from the bone marrow and lymph nodes into tissues. This receptor also controls brain endothelial barrier function, tight junctions, and permeability. In addition, S1P5 decreases NFKB activation on brain endothelial cells by lowering expression of adhesion molecules, inflammatory chemokines, and cytokines.4











.svg)