This site is intended for Healthcare Professionals only. if you are a member of the general public, click here
ZEPOSIA dosing schedule1
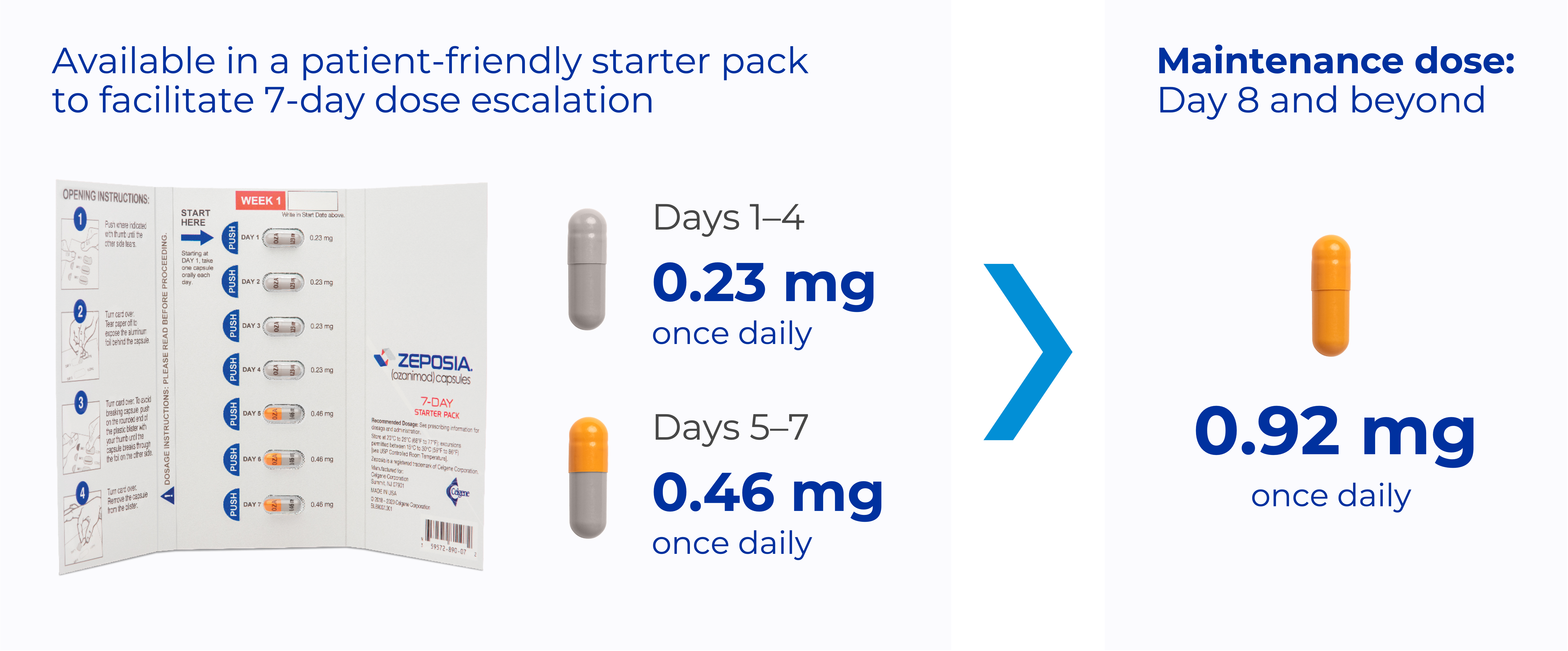
 Pinch & zoom to explore
Pinch & zoom to explore
No dose adjustment is necessary for patients with renal impairment. Patients with mild or moderate chronic hepatic impairment (Child-Pugh class A or B) are recommended to complete the 7-day dose escalation regimen, and then take 0.92 mg once every other day. ZEPOSIA was not evaluated in patients with severe hepatic impairment. Therefore, patients with severe hepatic impairment (Child-Pugh class C) must not be treated with ZEPOSIA.1
Pre-initiation and maintenance monitoring requirements
Pre-initiation1
One-time assessment:
- ECG to rule out any pre-existing heart conditions*
Standard assessments:
- Recent complete blood cell count (within last 6 months)
- Lymphocyte count
- Liver transaminase and bilirubin levels
- Exclude contraindications and clarify concomitant medications
- Confirm a negative pregnancy test in women of childbearing potential
Assessments for specific patients:
- Evaluate for signs of pre-existing macular oedema in patients with diabetes mellitus, uveitis or a history of retinal disease. An exam conducted with any optician is sufficient
- Administer live attenuated immunisations (if required) at least 1 month prior to initiation of ZEPOSIA
- VZV vaccination of patients without documented immunity to VZV is recommended
- Should be avoided during treatment and for 3 months after
Ongoing monitoring1
Maintenance assessments:
- Complete blood cell count done periodically throughout treatment
- Lymphocyte count
- Liver transaminase and bilirubin levels at Months 1, 3, 6, 9 and 12 on therapy and periodically thereafter
- Regularly monitor blood pressure during treatment
Assessments for specific patients:
- Re-evaluation of macular oedema in patients with diabetes mellitus, uveitis or a history of retinal disease during treatment. An exam conducted with any optician is sufficient
*First dose, hourly monitoring over a 6-hour period is recommended for patients with a resting heart rate <55 bpm, second-degree (Mobitz type I) AV block or a history of myocardial infarction or heart failure. These patients should be monitored for signs and symptoms of bradycardia.1
Please refer to the ZEPOSIA Summary of Product Characteristics for full safety information.
AV: atrioventricular; bpm: beats per minute; ECG, electrocardiogram; VZV, varicella-zoster virus
Reference
- ZEPOSIA (ozanimod) Summary of Product Characteristics, 2023.




