This site is intended for Healthcare Professionals only. if you are a member of the general public, click here
Post-hoc analysis: Clinical response** and remission* was observed in both TNFi-naive and TNFi-exposed patients starting at 4 weeks1,2
Clinical remission*
- Clinical remission was observed in both TNFi-naive and TNFi-exposed patients on ZEPOSIA at weeks 4 and 8, respectively2
Clinical response**
- Clinical response was observed in both TNFi-naive and TNFi-exposed patients on ZEPOSIA at weeks 4 and 8, respectively2
Bleeding
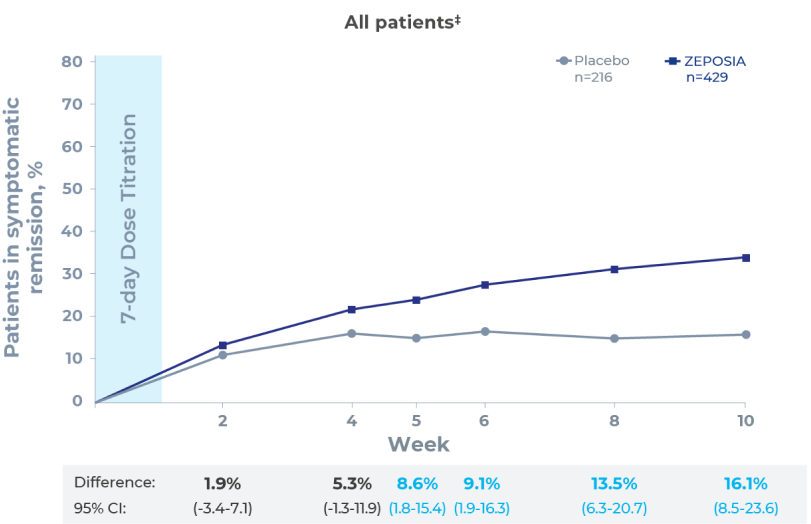
- With ZEPOSIA there was an observed reduction in rectal bleeding and stool frequency at 2 weeks (post-hoc analysis)1,3
This was a post-hoc analysis and should be interpreted with caution. The results for individual subgroups are not powered to draw meaningful conclusions and should therefore be interpreted with caution.
*Clinical remission was assessed using the three-component Mayo score and defined as a rectal-bleeding subscore of 0; a stool-frequency subscore of 1 or less, with a decrease of at least 1 point from baseline; and an endoscopy subscore of 1 or less (all on scales from 0 [none] to 3 [most severe]).3
**Clinical response was defined as a reduction in the total Mayo score of ≥3 points and ≥30% from baseline or in the three-component Mayo score of ≥2 points and ≥35% from baseline, as well as a reduction in the rectal-bleeding subscore of ≥1 point or an absolute rectal-bleeding subscore of ≤1 point.3
TNFi, tumour necrosis factor inhibitor; UC, ulcerative colitis.
References
- Sandborn WJ et al. NEJM 2021; 385(14):1280–1291.
- Siegmund B et al. Presented at: Congress of the European Crohn’s and Colitis Organisation Virtual Meeting; February 16–19, 2022.
- Zeposia (ozanimod) Summary of Product Characteristics, 2022.

 Pinch & zoom to explore
Pinch & zoom to explore