This site is intended for Healthcare Professionals only. if you are a member of the general public, click here
ZEPOSIA demonstrated a significant improvement in
clinical remission* at 10 weeks and 52 weeks vs. placebo (primary endpoint)1
You are advised to read the study design information before these efficacy outcomes
Primary endpoint: Clinical remission at week 10 (induction period) and at week 52 (maintenance period)1*

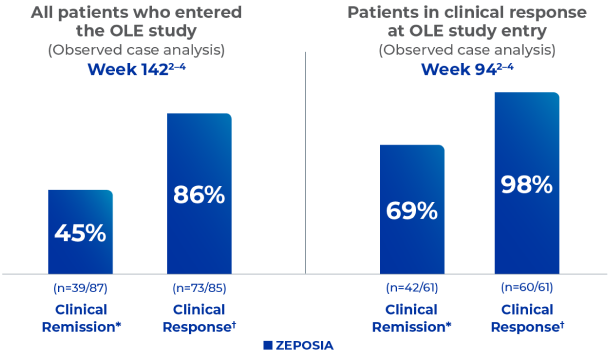
 Pinch & zoom to explore
Pinch & zoom to explore
In the TRUE NORTH study:
- Significantly more patients achieved clinical remission vs. placebo at week 101
- Twice as many patients achieved clinical remission vs. placebo at 52 weeks1
Adapted from Sandborn WJ et al. 2021.
*Patients in cohort 1 were stratified by prior TNFi use and corticosteroid use prior to randomisation.
Please see the True North study design for more information.
†Clinical remission was assessed using the three-component Mayo score and defined as a rectal-bleeding subscore of 0; a stool-frequency subscore of 1 or less, with a decrease of at least 1 point from baseline; and an endoscopy subscore of 1 or less (all on scales from 0 [none] to 3 [most severe]).
TNFi, tumour necrosis factor inhibitor; UC, ulcerative colitis.
References
- Sandborn WJ et al. NEJM 2021; 385(14):1280–1291
- Zeposia (ozanimod) Summary of Product Characteristics, 2022.
- Danese S et al. Long-term use of ozanimod in patients with moderately to severely active ulcerative colitis. Oral presentation at: European Crohn’s and Colitis Organisation (ECCO) Virtual, February 16-19, 2022. Presentation DOP44.
- Wolf DC et al. Long-term use of ozanimod in patients with moderately to severely active ulcerative colitis. Poster presented at: Digestive Disease Week (DDW); San Diego, CA, and Virtual, May 21-24, 2022. Poster Tu1458